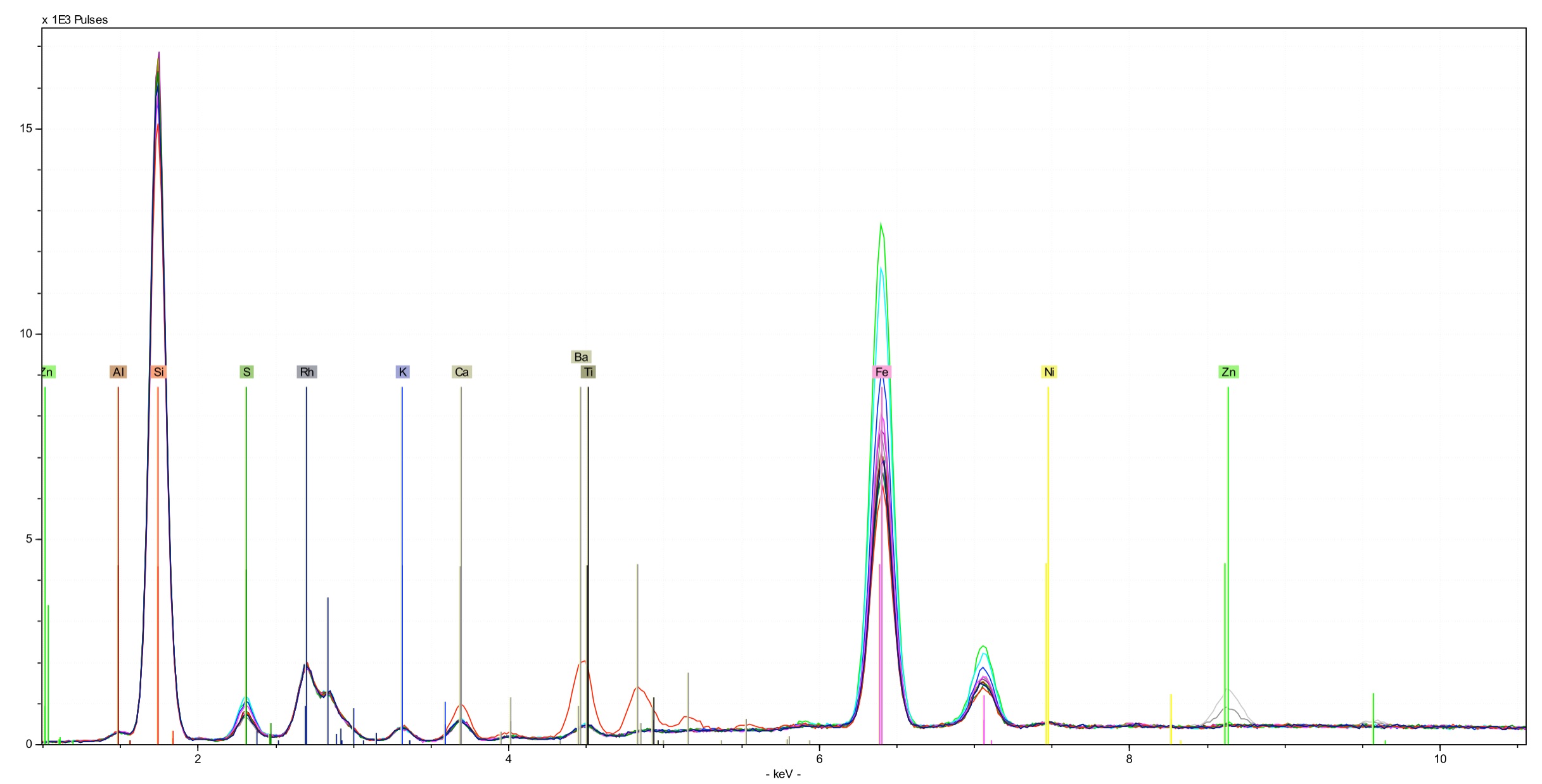
What is Fluorescence?
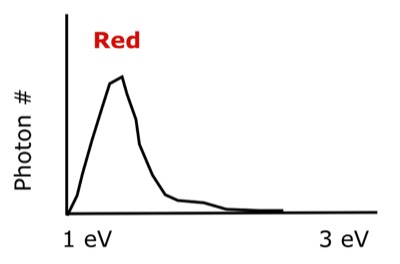
How do you see color? And what exactly is color? It is a fluorescence process, just like that used by the Tracer and the Titan. Red fluoresces at about 1.7 eV. Violet fluoresces a little above 3.1 eV. All the colors in between form a color spectrum. When you see the color red, it is really just a concentration of of photons with an energy higher than 1 eV, as depicted in the following figure.
How do you see color? And what exactly is color? It is a fluorescence process, just like that used by the Tracer and the Titan. Red fluoresces at about 1.7 eV. Violet fluoresces a little above 3.1 eV. All the colors in between form a color spectrum. When you see the color red, it is really just a concentration of of photons with an energy higher than 1 eV, as depicted in the following figure.
Color is not a fundamental property of matter, however - fluorescence is dependent upon a light source and a detector. For example, light from the sun can be thought of as a bell curves with its hump in the visible light spectra. Photons on the left tail have an energy at or less than 1 eV, these are infrared photons (heat). Photons with greater than 3.1 eV on the right tail are ultraviolet. Thus, the sun doesn't just give us light, but also gives us heat and sunburns. Incandescent lights were not like sunlight - they had far too many photons in the infrared (heat) portion of the spectrum and were inefficient. They almost had more in common with burners on an electric stove than with most other bulbs (photon excitation is due to resistance). Fluorescent light bulbs produce a bell curve like light from the sun, but with the tails stretching out less into the infrared and ultraviolet portions of the spectrum. Just as the energy range of the light sources is different, so too are the ways colors are perceived. We tend to look more pale in fluorescent light, and more red in incandescent light, than we do in the sunlight. In the same way, different X-ray tubes and filters will reveal elements in different ways.
At this time, it is worth considering in greater depth what occurs inside the tube, since so much is riding on it. A fluorescent lightbulb and X-ray tube are (almost) identical. In both tubes, electrons pour into one end of the tube from a wire. In both tubes, those electrons are attracted to a positive anode. Along their journey to the positive anode, they run into a metal. In fluorescent light bulbs, this is mercury, and it results in the excitation of photons in energy ranges from above 1 eV to 3 eV. We perceive this as white light. In X-ray tubes, the metal is rhodium, and instead of 1 - 3 eV photons being excited, we excite photons from 1,000 - 40,000 eV (1 - 40 keV).
At this time, it is worth considering in greater depth what occurs inside the tube, since so much is riding on it. A fluorescent lightbulb and X-ray tube are (almost) identical. In both tubes, electrons pour into one end of the tube from a wire. In both tubes, those electrons are attracted to a positive anode. Along their journey to the positive anode, they run into a metal. In fluorescent light bulbs, this is mercury, and it results in the excitation of photons in energy ranges from above 1 eV to 3 eV. We perceive this as white light. In X-ray tubes, the metal is rhodium, and instead of 1 - 3 eV photons being excited, we excite photons from 1,000 - 40,000 eV (1 - 40 keV).